Combustion Reactions

In a combustion reaction a hydrocarbon reacts with oxygen to form carbon dioxide and water.
Specifically in a combustion engine isooctane (C8H18) With 12.5 diatomic oxygen molecules. The balanced reaction is written below:
When the hydrocarbon isooctane overcomes the activation energy of the combustion reaction (by the spark plug) it reacts with the diatomic oxygen in the air. The oxygen is a gas and as we all know gasoline is a volatile liquid (Gasoline has more substances including addatives depending on what the engine is used for this is just a basic reaction). The energy goes into the isooctance and diatomic oxygen to severe the bonds. Lewis structures and a chart of bond enthalpies are below.



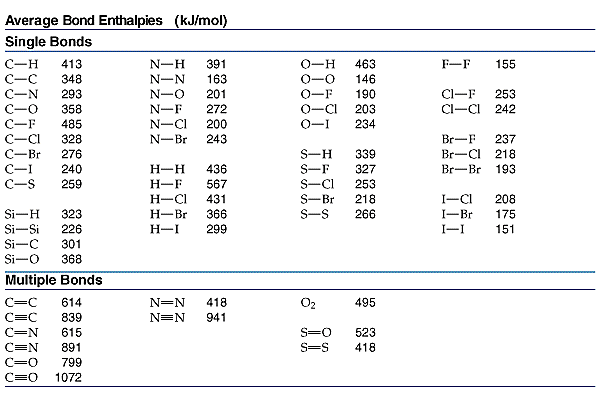
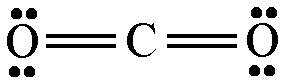
Title.click me.
Mathing It: Calculating the change in bond energy
Bonds broken-Bonds formed= delta H
-
18 C to H bonds per mole iooctane 2 moles of iooctane per reaction
-
7 C to C bonds per mole iooctane 2 moles of isooctane per reaction
-
1 double O to O bond per mole O2 25 moles O2 per reaction.
-
2 O to H bonds per mole water 18 moles of water per reaction
-
2 double bonded C to O bonds per mole 16 moles of carbon dioxide per reaction
(36(413) + 14(348) + 25(495)) - (32(799) + 36(463))= delta H
Delta H= -10,121 Kj/ balanced reaction.
The negative sign indicates that heat is released in this process, this heat energy is turned into mechanical energy in a combustion engine. The pressure created from this process is what powers the pistons.
The chart depicted left is of an energy versus reaction progression chart. This is a general representation of what happens in an exothermic reaction such as combustion. The products formed are lower in energy than the reactants, thus energy is released as the reaction progresses. That energy is then used to do work.

Title. Double click me.
Title. Double click me.
Visual Representation of Exothermic Reaction
*******Notice the increase in moles of gas
